
 |
|||||

| Tracks 1-20 | ||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-2
![]() DR LOVE: Would you review some of the key Gynecologic Oncology
Group trials in ovarian cancer?
DR LOVE: Would you review some of the key Gynecologic Oncology
Group trials in ovarian cancer?
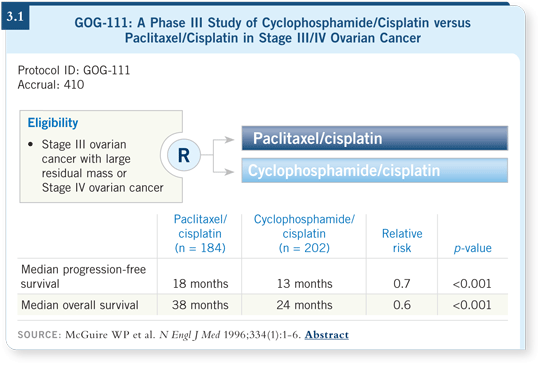
![]() DR HERZOG: The GOG has published some landmark trials in the last 11
years. The first one that made a difference in practice was GOG-111, which
substituted paclitaxel in place of cyclophosphamide in the cyclophosphamide/cisplatin regimen. A single-agent substitution resulted in a significant
improvement in overall survival (McGuire 1996; [3.1]).
DR HERZOG: The GOG has published some landmark trials in the last 11
years. The first one that made a difference in practice was GOG-111, which
substituted paclitaxel in place of cyclophosphamide in the cyclophosphamide/cisplatin regimen. A single-agent substitution resulted in a significant
improvement in overall survival (McGuire 1996; [3.1]).
This study evaluated bulk-disease Stage III and Stage IV ovarian cancer. Remarkably, the median survival time went from 24 months to 38 months simply with that single-agent substitution. This introduced the taxane era into clinical practice for ovarian cancer.
The next landmark trial was GOG-158 (Ozols 2003). This trial was limited to patients with Stage III disease that was optimally debulked with less than 1-cm residual disease as the largest tumor after primary surgery.
They were randomly assigned to the winner of GOG-111 — an inpatient paclitaxel/cisplatin regimen in which the paclitaxel was administered over 24 hours — or a much more user-friendly outpatient regimen consisting of carboplatin with only a three-hour paclitaxel infusion.
No statistical difference appeared between the two arms (3.2). In fact, patients in the carboplatin arm fared a little better than those in the cisplatin arm.

Track 5
![]() DR LOVE: What other advances have occurred through the GOG and
other research entities?
DR LOVE: What other advances have occurred through the GOG and
other research entities?
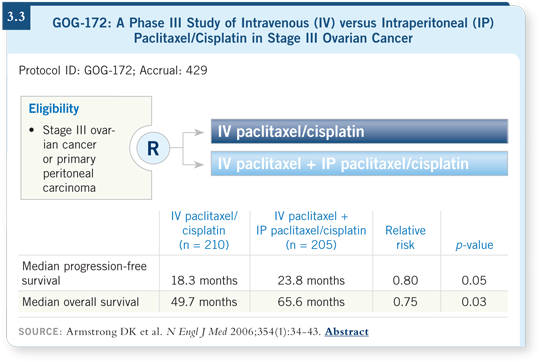
![]() DR HERZOG: The GOG-172 trial evaluated the role of administering a
portion of the therapy intraperitoneally. We saw a median survival of 66
months for patients with Stage III disease who received a component of their
care intraperitoneally versus 50 months for those who received only intravenous
medication (Armstrong 2006; [3.3]).
DR HERZOG: The GOG-172 trial evaluated the role of administering a
portion of the therapy intraperitoneally. We saw a median survival of 66
months for patients with Stage III disease who received a component of their
care intraperitoneally versus 50 months for those who received only intravenous
medication (Armstrong 2006; [3.3]).
What does that tell you? First, that’s a big difference in survival — almost 17 months. Second, in Stage III ovarian cancer, we now have median survivals that eclipse five years, which is most encouraging.
![]() DR LOVE: What about bevacizumab and intraperitoneal therapy?
DR LOVE: What about bevacizumab and intraperitoneal therapy?
![]() DR HERZOG: That’s a question the GOG is trying to settle. We are substituting
intraperitoneal carboplatin for cisplatin to see if we can reduce toxicity
and make the regimen more user friendly. Then we’re adding bevacizumab
into that equation, so we’re expecting a three-arm trial.
DR HERZOG: That’s a question the GOG is trying to settle. We are substituting
intraperitoneal carboplatin for cisplatin to see if we can reduce toxicity
and make the regimen more user friendly. Then we’re adding bevacizumab
into that equation, so we’re expecting a three-arm trial.
Track 16
![]() DR LOVE: Do you see bevacizumab fitting into therapy off protocol right
now?
DR LOVE: Do you see bevacizumab fitting into therapy off protocol right
now?
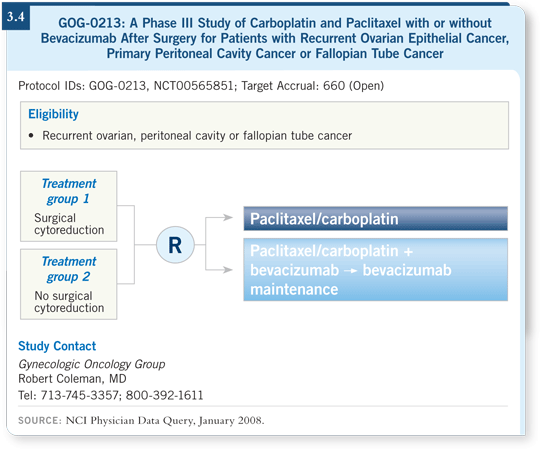
![]() DR HERZOG: The GOG-0213 trial was recently activated in the recurrent
setting. This trial is complex in that it’s trying to answer two questions in
recurrent, platinum-sensitive ovarian cancer (3.4).
DR HERZOG: The GOG-0213 trial was recently activated in the recurrent
setting. This trial is complex in that it’s trying to answer two questions in
recurrent, platinum-sensitive ovarian cancer (3.4).
First, should you take these patients to the operating room and debulk the disease again? Patients who are candidates for surgery are randomly assigned to surgery or no surgery.
The second question is, what’s the role of bevacizumab in that setting? Patients will be randomly assigned to carboplatin/paclitaxel alone or with bevacizumab followed by maintenance bevacizumab until disease progression. There’s no defined endpoint to the bevacizumab.
Off protocol, we’re seeing a lot of bevacizumab use. People are using it mostly in the recurrent setting based on the GOG-170-D data (Burger 2005). We are also seeing it used as a single agent, which has shown a 20-plus percent response rate.
![]() DR LOVE: If someone with recurrent disease came to you for a second
opinion, and she’d had bevacizumab recommended as a single agent, how
would you respond?
DR LOVE: If someone with recurrent disease came to you for a second
opinion, and she’d had bevacizumab recommended as a single agent, how
would you respond?
![]() DR HERZOG: It depends on where the patient is in the treatment queue, what
she’s received before and what kind of toxicities she’s developed, but largely, I
would agree with that recommendation.
DR HERZOG: It depends on where the patient is in the treatment queue, what
she’s received before and what kind of toxicities she’s developed, but largely, I
would agree with that recommendation.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Robert F Ozols, MD, PhD
- Select publications
Maurie Markman, MD
- Select publications
Thomas J Herzog, MD
- Select publications
Ovarian Cancer Update:
A CME Audio Series and Activity