
 |
|||||

| Tracks 1-20 | ||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 3-4
![]() DR LOVE: Can you review the key major trials in advanced ovarian
cancer?
DR LOVE: Can you review the key major trials in advanced ovarian
cancer?
![]() DR OZOLS: In a large Phase III trial conducted by the Gynecologic Oncology
Group (GOG-158), carboplatin/paclitaxel was shown to be superior to cisplatin/paclitaxel and became the standard regimen (Ozols 2000). Patients do experience hair loss and some neuropathy with this regimen, but it’s effective
and has a relatively favorable toxicity profile.
DR OZOLS: In a large Phase III trial conducted by the Gynecologic Oncology
Group (GOG-158), carboplatin/paclitaxel was shown to be superior to cisplatin/paclitaxel and became the standard regimen (Ozols 2000). Patients do experience hair loss and some neuropathy with this regimen, but it’s effective
and has a relatively favorable toxicity profile.
Studies were then conducted to determine whether adding a third drug to this regimen was beneficial, but a large GOG study (GOG0182-ICON5) with approximately 4,000 patients reported at ASCO showed that none of the three-drug regimens evaluated were better than the combination of paclitaxel and carboplatin (Bookman 2006).
Ongoing trials are comparing other combinations to paclitaxel/carboplatin, and additional studies have added a third drug, such as epirubicin (du Bois 2006), but none of them have been shown to improve survival as yet, compared to the gold standard.
![]() DR LOVE: What do we know about biologic therapy in the treatment of
ovarian cancer?
DR LOVE: What do we know about biologic therapy in the treatment of
ovarian cancer?
![]() DR OZOLS: The GOG conducted a Phase II trial (GOG-170-D) of single-agent
bevacizumab for previously treated patients with recurrent ovarian cancer,
and the response rate was nearly 20 percent, and more than 35 percent of the
patients did not have disease progression at six months (Burger 2005; [1.1]).
DR OZOLS: The GOG conducted a Phase II trial (GOG-170-D) of single-agent
bevacizumab for previously treated patients with recurrent ovarian cancer,
and the response rate was nearly 20 percent, and more than 35 percent of the
patients did not have disease progression at six months (Burger 2005; [1.1]).
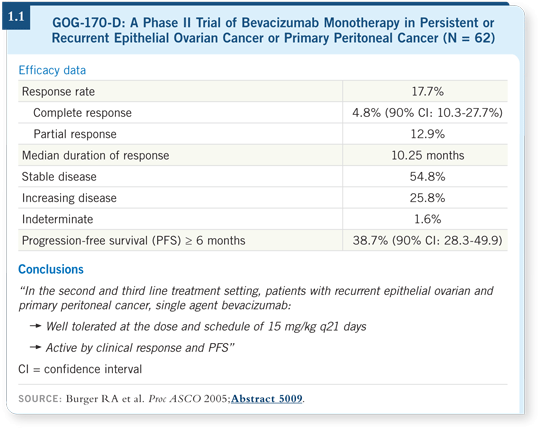
These data are striking because with other solid tumors, bevacizumab has been approved only in combination with chemotherapy. As a single agent, bevacizumab doesn’t have much activity in breast, lung or colorectal cancer, but in ovarian cancer it appears to be particularly active. Consequently, a good deal of interest has emerged in combining it with chemotherapy because, theoretically, it would potentiate the effects of chemotherapy as it does in the other tumor types, in addition to having its own activity.
Another interesting finding in the GOG-170-D trial was that no cases of gastrointestinal perforations were recorded. In a subsequent trial, approximately 10 percent of patients experienced this toxicity (Cannistra 2007), and if we pool all the data, it appears to occur in about five percent of patients.
Track 5
![]() DR LOVE: Can you discuss the data from the GOG-0218 clinical trial
evaluating bevacizumab combined with chemotherapy for previously
untreated patients with advanced ovarian or primary peritoneal cancer?
DR LOVE: Can you discuss the data from the GOG-0218 clinical trial
evaluating bevacizumab combined with chemotherapy for previously
untreated patients with advanced ovarian or primary peritoneal cancer?
![]() DR OZOLS: The GOG-170-D trial of single-agent bevacizumab resulted in
the launching of this Phase III trial in which patients are randomly assigned
to paclitaxel/carboplatin with a placebo versus paclitaxel/carboplatin with
concurrent bevacizumab versus paclitaxel/carboplatin/bevacizumab followed
by maintenance bevacizumab for 14 months (1.2).
DR OZOLS: The GOG-170-D trial of single-agent bevacizumab resulted in
the launching of this Phase III trial in which patients are randomly assigned
to paclitaxel/carboplatin with a placebo versus paclitaxel/carboplatin with
concurrent bevacizumab versus paclitaxel/carboplatin/bevacizumab followed
by maintenance bevacizumab for 14 months (1.2).

This trial is evaluating whether bevacizumab potentiates the effects of chemotherapy and whether it is beneficial continued as maintenance therapy.
Track 7
![]() DR LOVE: What are your thoughts about administering bevacizumab for
recurrent ovarian cancer in clinical practice?
DR LOVE: What are your thoughts about administering bevacizumab for
recurrent ovarian cancer in clinical practice?
![]() DR OZOLS: Bevacizumab is not approved for recurrent ovarian cancer, but
it is used quite extensively in clinical situations. For patients who have ascites
or pleural effusions, we’ve seen dramatic responses in improving these conditions
— even if the solid tumor doesn’t shrink — consequently sparing these
patients a paracentesis or thoracentesis. That’s an important quality-of-life
issue, and I believe bevacizumab will be a valuable adjunct to the treatment of
such patients.
DR OZOLS: Bevacizumab is not approved for recurrent ovarian cancer, but
it is used quite extensively in clinical situations. For patients who have ascites
or pleural effusions, we’ve seen dramatic responses in improving these conditions
— even if the solid tumor doesn’t shrink — consequently sparing these
patients a paracentesis or thoracentesis. That’s an important quality-of-life
issue, and I believe bevacizumab will be a valuable adjunct to the treatment of
such patients.
We’ve had patients who aren’t interested in participating in the GOG-0218 trial because they want to receive bevacizumab and don’t want to take a chance of being assigned to a placebo arm. If the patient understands the cost and the risks that have been associated with this treatment, I believe using it in clinical practice is a reasonable approach.
In that setting, I would use bevacizumab concurrently with the chemotherapy because that’s how it’s been shown to be effective against other solid tumors. Also, given the long stabilization of disease observed in many patients in the GOG single-agent trial, I would continue it as maintenance therapy, if the patient was tolerating treatment and cost was not an issue.
Track 9
![]() DR LOVE: What are the usual approaches to recurrent ovarian cancer?
DR LOVE: What are the usual approaches to recurrent ovarian cancer?
![]() DR OZOLS: For patients with platinum-sensitive disease, two regimens are
currently being used. One is gemcitabine and carboplatin, which was recently
approved by the FDA for platinum-sensitive disease on the basis of data from
a randomized trial in Europe — a trial that evaluated carboplatin with or
without gemcitabine and showed a three-month improvement in progression-free
survival with the combination (Pfisterer 2006).
DR OZOLS: For patients with platinum-sensitive disease, two regimens are
currently being used. One is gemcitabine and carboplatin, which was recently
approved by the FDA for platinum-sensitive disease on the basis of data from
a randomized trial in Europe — a trial that evaluated carboplatin with or
without gemcitabine and showed a three-month improvement in progression-free
survival with the combination (Pfisterer 2006).
The other is paclitaxel and carboplatin. An earlier study — conducted by the ICON investigators in Europe, which evaluated carboplatin with or without paclitaxel in platinum-sensitive recurrent disease — likewise showed an improvement of three months in progression-free survival and a slight improvement in overall survival (Parmar 2003) with the combination.
In terms of efficacy, I believe these two regimens are equal. The difference is primarily in toxicity. With paclitaxel/carboplatin, patients experience hair loss, and if a patient is experiencing neuropathy from prior therapy, this regimen may exacerbate that toxicity. While gemcitabine/carboplatin doesn’t exacerbate neuropathy, it is associated with more myelosuppression, but oncologists who use this combination frequently know how to minimize the consequences of this toxicity. This regimen is being used more frequently, and trials are now adding bevacizumab to see if that improves efficacy.
![]() DR LOVE: What do we know about the role of anti-HER2 agents, such as
trastuzumab and pertuzumab, in the treatment of recurrent ovarian cancer?
DR LOVE: What do we know about the role of anti-HER2 agents, such as
trastuzumab and pertuzumab, in the treatment of recurrent ovarian cancer?
![]() DR OZOLS: We initially thought that the overexpression of HER2 would be
common in ovarian tumors, but in a large study, the GOG found that only
approximately 10 percent of patients had disease that overexpressed HER2,
and only a couple of responses to trastuzumab were recorded (Bookman
2003a). So trastuzumab by itself is not generally considered an active agent for
ovarian cancer.
DR OZOLS: We initially thought that the overexpression of HER2 would be
common in ovarian tumors, but in a large study, the GOG found that only
approximately 10 percent of patients had disease that overexpressed HER2,
and only a couple of responses to trastuzumab were recorded (Bookman
2003a). So trastuzumab by itself is not generally considered an active agent for
ovarian cancer.
Pertuzumab is a monoclonal antibody that binds several sites on the different EGFR families. It’s still too early to tell whether it will be a useful drug in ovarian cancer, but some responses were reported with it as monotherapy (Gordon 2006).
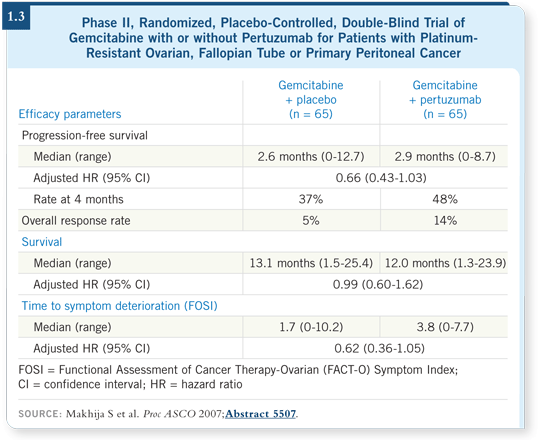
In 2007, data were presented at ASCO from a Phase II trial of gemcitabine with or without pertuzumab, and the combination appeared to have substantial activity (Makhija 2007; [1.3]). While the responses were relatively low, the data may be encouraging enough to continue a larger study. Also, there may be some markers for response to this regimen, so that study will be expanded.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Robert F Ozols, MD, PhD
- Select publications
Maurie Markman, MD
- Select publications
Thomas J Herzog, MD
- Select publications
Ovarian Cancer Update:
A CME Audio Series and Activity