
 |
|||||

| Tracks 1-22 | ||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you discuss some of the important ongoing clinical
trials in ovarian cancer?
DR LOVE: Would you discuss some of the important ongoing clinical
trials in ovarian cancer?
![]() DR ARMSTRONG: Probably the most provocative and interesting ongoing
studies are incorporating bevacizumab. It is interesting that unlike the diseases
for which bevacizumab has FDA approval — lung, colorectal and breast cancer
— bevacizumab has strikingly significant single-agent activity in ovarian
cancer.
DR ARMSTRONG: Probably the most provocative and interesting ongoing
studies are incorporating bevacizumab. It is interesting that unlike the diseases
for which bevacizumab has FDA approval — lung, colorectal and breast cancer
— bevacizumab has strikingly significant single-agent activity in ovarian
cancer.
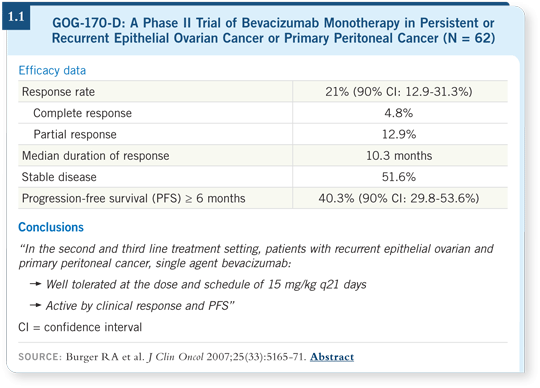
I believe that in renal cell cancer, the response rate with bevacizumab may be nine or 10 percent, but in the single-agent study by the Gynecologic Oncology Group (GOG) in ovarian cancer, the response rate was 21 percent (Burger 2007; [1.1]).
If we obtain the same results that we’ve seen in some of the other diseases, in which bevacizumab seems to make chemotherapy more effective, we may be able to extend the current limits of treatment for ovarian cancer.
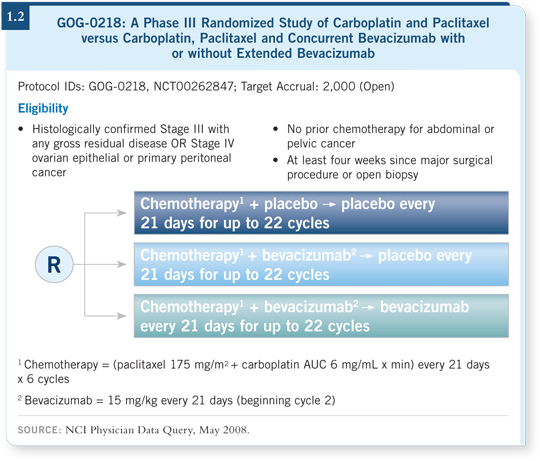
The GOG-0218 trial for patients with newly diagnosed ovarian cancer is a randomized study evaluating paclitaxel/carboplatin alone, paclitaxel/carboplatin with bevacizumab and paclitaxel/carboplatin with bevacizumab and bevacizumab consolidation.
All three arms of the trial have patients coming in every three weeks after the completion of chemotherapy to receive either placebo or bevacizumab (1.2).
In ovarian cancer, we have the fortunate situation that even with bulky disease, most patients respond to chemotherapy. If bevacizumab performs as it does in other diseases, we might be changing the paradigm for treatment of this disease.
Track 12
![]() DR LOVE: What do we know about response rates and disease control
with the available treatment options for patients who experience a recurrence
within six months after receiving initial therapy with a platinum
agent and a taxane? What is the role of re-treating with a platinum agent?
DR LOVE: What do we know about response rates and disease control
with the available treatment options for patients who experience a recurrence
within six months after receiving initial therapy with a platinum
agent and a taxane? What is the role of re-treating with a platinum agent?
![]() DR ARMSTRONG: For clinical trials, we have used recurrence within six
months as our cutoff to define platinum-resistant and platinum-sensitive disease.
Unfortunately, that has probably led to the magical thinking that something
unique and wonderful happens at six months, but actually it’s a continuum.
DR ARMSTRONG: For clinical trials, we have used recurrence within six
months as our cutoff to define platinum-resistant and platinum-sensitive disease.
Unfortunately, that has probably led to the magical thinking that something
unique and wonderful happens at six months, but actually it’s a continuum.
If you evaluate any of the available strategies — topotecan, liposomal doxorubicin, re-treatment with a platinum or a taxane — the farther out the patient is from the initial therapy, the greater the sensitivity and the higher the response rate. The time from completion of initial therapy is a measure of potential chemotherapy sensitivity.
We don’t see huge response rates with almost anything used in the first six months. Obtaining another complete response is difficult when the patient experiences a recurrence within the first six to 12 months. It happens, but it’s not common. The longer someone is out from the initial therapy, the more likely we are to obtain a complete response.
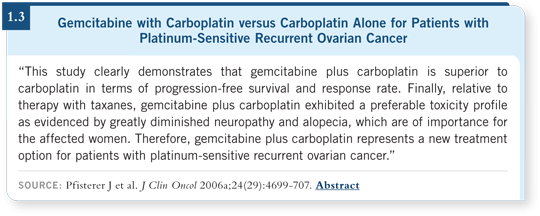
Two large randomized studies have evaluated a platinum agent alone versus platinum-based combinations for patients with late relapses — after six to 12 months. ICON-4 compared paclitaxel/carboplatin or cisplatin to carboplatin or cisplatin alone (Parmar 2003), and the AGO trial evaluated carboplatin versus gemcitabine/carboplatin (Pfisterer 2006a; [1.3]).
Both of those trials showed an improvement in progression-free survival with combination therapy. The gemcitabine/carboplatin versus carboplatin study was not powered to detect differences in overall survival, and it didn’t show any difference.
It’s nice to have more than one platinum-based combination to offer patients. I believe both of these studies have led to a paradigm shift. For patients whose disease recurs more than six to 12 months after initial therapy, most of us will reuse a platinum-based doublet.
![]() DR LOVE: For a patient who experiences a recurrence at six months, what
would you most likely consider?
DR LOVE: For a patient who experiences a recurrence at six months, what
would you most likely consider?
![]() DR ARMSTRONG: At six months, especially with measurable disease, I would use
sequential single agents. I would start with a nonplatinum agent.
DR ARMSTRONG: At six months, especially with measurable disease, I would use
sequential single agents. I would start with a nonplatinum agent.
Track 13
![]() DR LOVE: What’s the role of consolidation therapy?
DR LOVE: What’s the role of consolidation therapy?
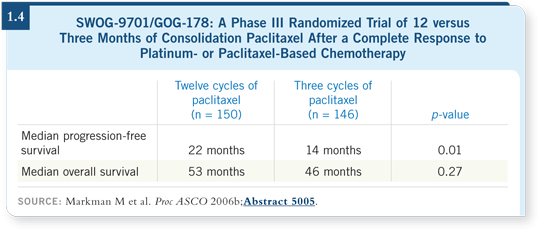
![]() DR ARMSTRONG: SWOG-9701/GOG-178 evaluated every four-week paclitaxel
for 12 or three cycles in patients with ovarian cancer who had received
five or six cycles of a platinum agent and a taxane and achieved a clinical
complete response.
DR ARMSTRONG: SWOG-9701/GOG-178 evaluated every four-week paclitaxel
for 12 or three cycles in patients with ovarian cancer who had received
five or six cycles of a platinum agent and a taxane and achieved a clinical
complete response.
The Data Safety Monitoring Board stopped the study at approximately half the projected accrual because an improvement in progression-free survival was evident for the patients who received 12 months of paclitaxel (Markman 2006b; [1.4]).
No survival advantage was observed. Is that because the trial was stopped early?
No one knows. Is the standard now almost 18 months of chemotherapy?
I believe that doctors should discuss it with the patient and make a decision about whether to continue with maintenance therapy. People have argued that it may be just as effective to wait until clinical relapse and then use the drugs. I tend not to use consolidation therapy with a taxane.
We have conducted two similar types of studies with a short duration of topotecan therapy, and neither has shown a benefit (Pfisterer 2006b; De Placido 2004). However, those trials used three months of therapy, which was the control arm in the paclitaxel study. Perhaps if we’d used a longer duration of therapy with topotecan, we would have observed some benefit.
I believe this is a situation in which the biologic agents will play a role instead of more chemotherapy. They would have less toxicity. It would be lovely if we had the equivalent of hormonal therapy in breast cancer. Most patients reach remission, but it doesn’t last.
Track 14
![]() DR LOVE: Here’s a quick case question for you: What do you say to a
patient who is asymptomatic with a stable CT after six cycles of pegylated
liposomal doxorubicin as second-line therapy?
DR LOVE: Here’s a quick case question for you: What do you say to a
patient who is asymptomatic with a stable CT after six cycles of pegylated
liposomal doxorubicin as second-line therapy?
![]() DR ARMSTRONG: I tell patients, “We will keep you on this therapy. We will
stop it for either of two reasons. One is if your disease progresses. The other is
if you develop intolerable toxicities.”
DR ARMSTRONG: I tell patients, “We will keep you on this therapy. We will
stop it for either of two reasons. One is if your disease progresses. The other is
if you develop intolerable toxicities.”
The bigger question is, if we stop the therapy and the patient hasn’t experienced a complete response, what is the chance that the tumor will grow quickly? If the tumor starts to grow, what is the chance that reintroducing the drug will induce a response again?
![]() DR LOVE: What do you think about continuing liposomal doxorubicin at the
same dose but perhaps for a longer interval?
DR LOVE: What do you think about continuing liposomal doxorubicin at the
same dose but perhaps for a longer interval?
![]() DR ARMSTRONG: Most of our patients tell us that the last week before they come in for chemotherapy is the week when they feel best. So you can argue
that by increasing the time between treatments, you will provide patients with
the best quality of life. I certainly have done that with patients for whom I had
concerns about nonhematologic toxicities for which you don’t necessarily have
to hold treatment.
DR ARMSTRONG: Most of our patients tell us that the last week before they come in for chemotherapy is the week when they feel best. So you can argue
that by increasing the time between treatments, you will provide patients with
the best quality of life. I certainly have done that with patients for whom I had
concerns about nonhematologic toxicities for which you don’t necessarily have
to hold treatment.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Deborah K Armstrong, MD
- Select publications
David R Spriggs, MD
- Select publications
Robert L Coleman, MD
- Select publications
Ovarian Cancer Update:
A CME Audio Series and Activity