
 |
|||||

| Tracks 1-13 | ||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 4-5
![]() DR LOVE: Would you discuss the GOG-0213 study?
DR LOVE: Would you discuss the GOG-0213 study?
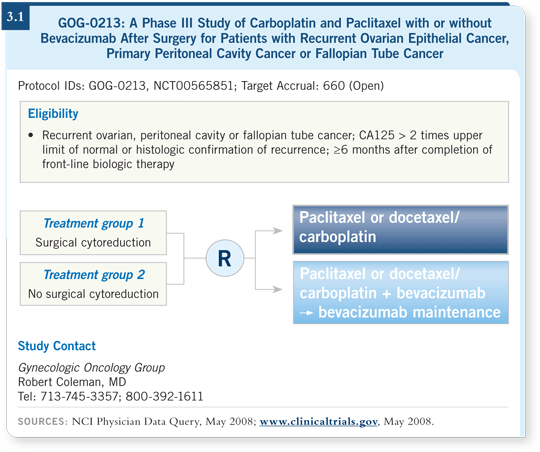
![]() DR COLEMAN: GOG-0213 will examine two key issues: surgery in recurrent disease and the role of adding biologic agents to standard chemotherapy in the recurrent setting (3.1). The trial will enroll patients with platinum-sensitive recurrent ovarian cancer treated with a prior regimen for recurrence, although the patients could have received maintenance therapy as part of the primary treatment. This trial also allows for prior exposure to biologic agents.
DR COLEMAN: GOG-0213 will examine two key issues: surgery in recurrent disease and the role of adding biologic agents to standard chemotherapy in the recurrent setting (3.1). The trial will enroll patients with platinum-sensitive recurrent ovarian cancer treated with a prior regimen for recurrence, although the patients could have received maintenance therapy as part of the primary treatment. This trial also allows for prior exposure to biologic agents.
Recently, we amended the trial to allow patients who have a biological recurrence, which has been defined as an elevation in CA125 to twice the upper limit of normal or greater than 100 u/mL after it normalizes. Platinum sensitivity in this trial is evaluated by traditional parameters at six months. We made the important decision to evaluate patients at six to 12 months and after 12 months in both the chemotherapy and the surgery arms. Some information suggests that patients with longer disease-free intervals may benefit more from surgery.
![]() DR LOVE: What’s the trial design?
DR LOVE: What’s the trial design?
![]() DR COLEMAN: The first randomization has to do with whether the patient is considered to be a surgical candidate. This was a particularly difficult parameter
to develop because surgeons have different criteria for what they consider operable in the recurrent setting.
DR COLEMAN: The first randomization has to do with whether the patient is considered to be a surgical candidate. This was a particularly difficult parameter
to develop because surgeons have different criteria for what they consider operable in the recurrent setting.
In prior retrospective and prospective nonrandomized reports, the only consistent observation was that patients with no visible residual disease after surgery seemed to benefit the most from this procedure. We defined surgical eligibility based on that endpoint. If an investigator felt that all of a patient’s disease could be removed, the patient was eligible to enter the surgical randomization.
After this randomization, or if patients are not surgical candidates, they go on to the chemotherapy randomization, which compares paclitaxel/carboplatin in the standard treatment arm to paclitaxel/carboplatin and bevacizumab, followed by bevacizumab maintenance, in the experimental arm.
Track 9
![]() DR LOVE: Would you discuss the findings of your recent paper on CA125?
DR LOVE: Would you discuss the findings of your recent paper on CA125?
![]() DR COLEMAN: We evaluated a data set from the pegylated liposomal doxorubicin
registration trial (Gordon 2001), which provided a unique window into the short period during the first two cycles of therapy. We wanted to examine how CA125 performed among the responders and nonresponders in that cohort.
DR COLEMAN: We evaluated a data set from the pegylated liposomal doxorubicin
registration trial (Gordon 2001), which provided a unique window into the short period during the first two cycles of therapy. We wanted to examine how CA125 performed among the responders and nonresponders in that cohort.
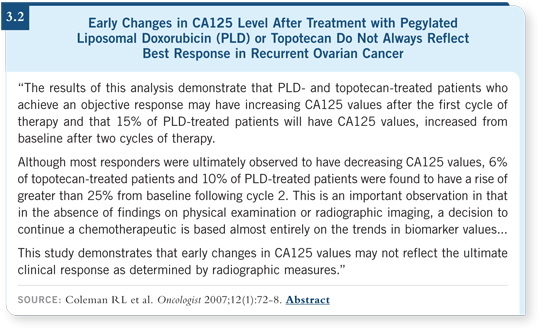
We observed a large number of responders with rising CA125 levels after they began therapy (Coleman 2007; [3.2]). Because of the reliance on CA125 in the community, I was afraid that people were abandoning the drugs early in therapy because of these effects in CA125 levels.
It’s important to realize that you can observe a rise in CA125 early on in treatment. Don’t have a knee-jerk reaction and assume that the patients will not benefit from therapy.
Track 13
![]() DR LOVE: What is your approach to systemic therapy of platinum-sensitive
recurrent disease?
DR LOVE: What is your approach to systemic therapy of platinum-sensitive
recurrent disease?
![]() DR COLEMAN: Traditionally, platinum sensitivity describes the time during which patients who have received a platinum again would likely have a response, which is approximately six months (Blackledge 1989; Markman 1991). We expect the phenotype of platinum sensitivity to extend across all drugs in that most drugs perform better in patients with longer treatment-free intervals. A near linear relationship exists between those two.
DR COLEMAN: Traditionally, platinum sensitivity describes the time during which patients who have received a platinum again would likely have a response, which is approximately six months (Blackledge 1989; Markman 1991). We expect the phenotype of platinum sensitivity to extend across all drugs in that most drugs perform better in patients with longer treatment-free intervals. A near linear relationship exists between those two.
Having said that, we tend to treat platinum-sensitive disease with combination therapy. Both paclitaxel/carboplatin and newly approved gemcitabine/carboplatin are important combinations to consider in that setting. A meta-analysis presented at ASCO suggests that these combinations tend to help patients in the short term (Orlando 2007).
The major question is whether sequencing the same agents will yield the same endpoint. That’s the focus of a trial run by Dr Angeles Alvarez Secord at Duke, who’s evaluating docetaxel and carboplatin administered either in combination or as sequential therapy (NCT00090610). We may find that the combination of these agents adds toxicity and short-term gains but adds nothing in terms of long-term survival.
![]() DR LOVE: How do you approach treatment for these patients in the clinical setting?
DR LOVE: How do you approach treatment for these patients in the clinical setting?
![]() DR COLEMAN: I tend to offer patients the most aggressive therapy that I feel is safe. If these patients are good candidates for surgery, with resectable disease, I consider them for surgery.
DR COLEMAN: I tend to offer patients the most aggressive therapy that I feel is safe. If these patients are good candidates for surgery, with resectable disease, I consider them for surgery.
Off protocol and without surgery considerations, if the toxicities are not too high, I recommend reinduction with a taxane/platinum regimen or with gemcitabine/carboplatin, particularly for patients with neurotoxicity from their first-line therapy.
![]() DR LOVE: What have you observed with gemcitabine/carboplatin in terms of tolerance and antitumor effects?
DR LOVE: What have you observed with gemcitabine/carboplatin in terms of tolerance and antitumor effects?
![]() DR COLEMAN: It’s quite tolerable. Gemcitabine is approved for weekly administration.
To make that administration safe, we use a lower dose of the carboplatin.
Gemcitabine/cisplatin is also a reasonable strategy for this tumor type.
DR COLEMAN: It’s quite tolerable. Gemcitabine is approved for weekly administration.
To make that administration safe, we use a lower dose of the carboplatin.
Gemcitabine/cisplatin is also a reasonable strategy for this tumor type.
One modification we’ve made for patients who are intolerant to gemcitabine/carboplatin or gemcitabine/cisplatin on the traditional schedule is to break it up and administer both the platinum agent and the gemcitabine every 14 days. That’s well tolerated, and we’ve seen impressive responses.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Deborah K Armstrong, MD
- Select publications
David R Spriggs, MD
- Select publications
Robert L Coleman, MD
- Select publications
Ovarian Cancer Update:
A CME Audio Series and Activity