
 |
|||||

| Tracks 1-19 | ||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1, 5-7, 11-12
![]() DR LOVE: What was the rationale behind the Phase II trial combining intraperitoneal chemotherapy and systemic bevacizumab as first-line treatment
for optimal Stage II or Stage III ovarian cancer (2.1)?
DR LOVE: What was the rationale behind the Phase II trial combining intraperitoneal chemotherapy and systemic bevacizumab as first-line treatment
for optimal Stage II or Stage III ovarian cancer (2.1)?
![]() DR SPRIGGS: Memorial Sloan-Kettering has been committed to identifying ways to enhance the delivery of intraperitoneal chemotherapy for almost 20 years, and the evolution of the field has been quite extraordinary. In the past 10 to 12 years, three consecutive Phase III studies have demonstrated a substantial survival advantage for patients receiving intraperitoneal chemotherapy
for ovarian cancer (Alberts 1996; Markman 2001; Armstrong 2002).
DR SPRIGGS: Memorial Sloan-Kettering has been committed to identifying ways to enhance the delivery of intraperitoneal chemotherapy for almost 20 years, and the evolution of the field has been quite extraordinary. In the past 10 to 12 years, three consecutive Phase III studies have demonstrated a substantial survival advantage for patients receiving intraperitoneal chemotherapy
for ovarian cancer (Alberts 1996; Markman 2001; Armstrong 2002).
The peritoneal cavity is lined by a mesothelium that expresses mesothelin, a documented target and presumed ligand for the CA125/MUC16 molecule. We believe that because so many cases of ovarian cancer express CA125/MUC16, it has a propensity to bind to these tissues, and indeed, a predominance of ovarian cancer occurs in the peritoneal cavity. Therefore, delivering systemic therapy to the cavity is a rational approach.
We added bevacizumab systemically because it has significant activity in ovarian cancer. A series of reports have suggested that single-agent bevacizumab is potent in inhibiting tumor growth and even in shrinking ovarian tumors (Han 2007).
![]() DR LOVE: What was the rationale for the trial design?
DR LOVE: What was the rationale for the trial design?

![]() DR SPRIGGS: We know that a huge difference in toxicity exists between 75 and 100 mg/m2 of cisplatin and, based on the intravenous cisplatin data, we believe 50 is as effective as 100 mg/m2. By decreasing the intraperitoneal dose to 75 mg/m2, we felt we could ensure that approximately two thirds, or 50 mg/m2, of the cisplatin would get into the circulation, which is reported to be equivalent to 100 mg/m2 (McGuire 1995).
DR SPRIGGS: We know that a huge difference in toxicity exists between 75 and 100 mg/m2 of cisplatin and, based on the intravenous cisplatin data, we believe 50 is as effective as 100 mg/m2. By decreasing the intraperitoneal dose to 75 mg/m2, we felt we could ensure that approximately two thirds, or 50 mg/m2, of the cisplatin would get into the circulation, which is reported to be equivalent to 100 mg/m2 (McGuire 1995).
We knew that paclitaxel stays in the peritoneal cavity for a long time and felt the sustained exposure was probably important in the success of GOG-172, so we elected to use intraperitoneal paclitaxel (Armstrong 2002). Also, we chose to administer paclitaxel and cisplatin on separate days to avoid the neuropathy associated with combining these agents. So far, the level of neurotoxicity has been acceptable.
![]() DR LOVE: What have you observed with this regimen with regard to tolerability?
DR LOVE: What have you observed with this regimen with regard to tolerability?
![]() DR SPRIGGS: We’ve found that patients can tolerate the regimen. The dropout rate is not high, and the toxicities are modest (Konner 2007; [2.1]).
DR SPRIGGS: We’ve found that patients can tolerate the regimen. The dropout rate is not high, and the toxicities are modest (Konner 2007; [2.1]).
Bevacizumab is a remarkably well-tolerated drug. Understanding the physiology of the kidney and how VEGF works, it’s not surprising that some proteinuria occurs and that bleeding and clotting problems can occur, although the frequency is low. While hypertension is fairly common, none of these side effects are deal breakers.
The difficulty is that the first-line treatment of ovarian cancer includes an aggressive surgical procedure. We need to ensure that we are not promoting the formation of new blood clots, heart attacks or strokes in these patients. We also have the concern that bevacizumab might interfere with new blood vessel formation during wound healing.
Based on the colorectal cancer experience, our patients do not receive bevacizumab for six to eight weeks after surgery, and we have found that to be safe. The other issue is bowel perforation, which has been reported as a side effect of bevacizumab in patients treated for ovarian cancer (Cannistra 2007).
![]() DR LOVE: How do you use bevacizumab in your practice off study?
DR LOVE: How do you use bevacizumab in your practice off study?
![]() DR SPRIGGS: Bevacizumab has single-agent effectiveness of 15 to 20 percent (1.1, page 4), which is comparable to gemcitabine, topotecan and vinorelbine,
among other agents, so it’s a reasonable choice for patients who have received pegylated liposomal doxorubicin and gemcitabine as single agents for platinum-resistant disease. Bevacizumab has relatively few chronic side effects, but it does have some rare serious side effects, so we need to have a careful discussion with patients regarding the benefits and risks.
DR SPRIGGS: Bevacizumab has single-agent effectiveness of 15 to 20 percent (1.1, page 4), which is comparable to gemcitabine, topotecan and vinorelbine,
among other agents, so it’s a reasonable choice for patients who have received pegylated liposomal doxorubicin and gemcitabine as single agents for platinum-resistant disease. Bevacizumab has relatively few chronic side effects, but it does have some rare serious side effects, so we need to have a careful discussion with patients regarding the benefits and risks.
Tracks 13-14
![]() DR LOVE: What is your algorithm for first-line therapy after surgery?
DR LOVE: What is your algorithm for first-line therapy after surgery?
![]() DR SPRIGGS: At Memorial Sloan-Kettering, we first try to enroll patients in a clinical trial. In our up-front program, patients whose disease is optimally debulked are entered in the Phase II trial of intravenous and intraperitoneal paclitaxel with intraperitoneal cisplatin and intravenous bevacizumab. If the patient can’t receive bevacizumab but is a candidate for intraperitoneal therapy, we administer the same regimen but without bevacizumab off study.
DR SPRIGGS: At Memorial Sloan-Kettering, we first try to enroll patients in a clinical trial. In our up-front program, patients whose disease is optimally debulked are entered in the Phase II trial of intravenous and intraperitoneal paclitaxel with intraperitoneal cisplatin and intravenous bevacizumab. If the patient can’t receive bevacizumab but is a candidate for intraperitoneal therapy, we administer the same regimen but without bevacizumab off study.
Patients whose disease is suboptimally debulked are enrolled on GOG-0218 comparing carboplatin/paclitaxel to carboplatin/paclitaxel/bevacizumab with or without extended bevacizumab (1.2, page 5). Patients who cannot receive bevacizumab receive carboplatin and paclitaxel off study.
![]() DR LOVE: Once the disease progresses, how do you treat?
DR LOVE: Once the disease progresses, how do you treat?
![]() DR SPRIGGS: Most patients achieve a partial, if not a complete, remission with their primary therapy. Those who do not, but rather experience disease progression while receiving chemotherapy, represent an adverse group. Outside of a clinical trial, I would use pegylated liposomal doxorubicin, understanding that despite therapy, these patients still do not have a good prognosis.
DR SPRIGGS: Most patients achieve a partial, if not a complete, remission with their primary therapy. Those who do not, but rather experience disease progression while receiving chemotherapy, represent an adverse group. Outside of a clinical trial, I would use pegylated liposomal doxorubicin, understanding that despite therapy, these patients still do not have a good prognosis.
For the patients who do achieve a remission but then experience recurrence, we use the platinum-sensitivity mark that Markman put forward, by which patients whose disease recurs within six months represent the platinum resistant, and they are most likely to receive either an investigational agent in a Phase II trial or pegylated liposomal doxorubicin as the alternative (Markman 1992).
Track 15
![]() DR LOVE: How does the efficacy of pegylated liposomal doxorubicin compare to gemcitabine?
DR LOVE: How does the efficacy of pegylated liposomal doxorubicin compare to gemcitabine?
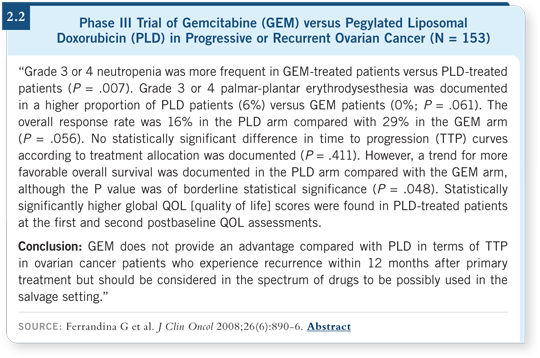
![]() DR SPRIGGS: A recent publication in the JCO suggests that pegylated liposomal doxorubicin is similar, not inferior, to gemcitabine in efficacy (Ferrandina 2008; [2.2]). However, it is important to remember that pegylated liposomal doxorubicin has a slow onset of action, and for a patient with rapidly progressive disease, we may not have time to wait for it to begin to work. In that setting, a topoisomerase inhibitor or gemcitabine is probably a better choice.
DR SPRIGGS: A recent publication in the JCO suggests that pegylated liposomal doxorubicin is similar, not inferior, to gemcitabine in efficacy (Ferrandina 2008; [2.2]). However, it is important to remember that pegylated liposomal doxorubicin has a slow onset of action, and for a patient with rapidly progressive disease, we may not have time to wait for it to begin to work. In that setting, a topoisomerase inhibitor or gemcitabine is probably a better choice.
Otherwise, pegylated liposomal doxorubicin has many advantages. We administer it once every four weeks, rather than every three, and our starting dose is 40 mg/m2 because we find that’s more tolerable than 50 mg/m2.
![]() DR LOVE: What is the toxicity profile?
DR LOVE: What is the toxicity profile?
![]() DR SPRIGGS: Some say they have never seen a true case of pegylated liposomal doxorubicin-related cardiotoxicity, but I have. The conservative approach is to order echocardiograms as you go beyond two or three cycles, and when you get to a maximum total doxorubicin dose in the 400-to 450-mg range, you should carefully weigh the benefit and risks. We don’t know exactly how much pegylated liposomal doxorubicin is enough.
DR SPRIGGS: Some say they have never seen a true case of pegylated liposomal doxorubicin-related cardiotoxicity, but I have. The conservative approach is to order echocardiograms as you go beyond two or three cycles, and when you get to a maximum total doxorubicin dose in the 400-to 450-mg range, you should carefully weigh the benefit and risks. We don’t know exactly how much pegylated liposomal doxorubicin is enough.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Deborah K Armstrong, MD
- Select publications
David R Spriggs, MD
- Select publications
Robert L Coleman, MD
- Select publications
Ovarian Cancer Update:
A CME Audio Series and Activity