
 |
|||||

| Tracks 1-12 | ||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1, 4-5
![]() DR LOVE: Can you discuss the data from the GOG-0170D trial evaluating
the use of bevacizumab for patients with persistent or recurrent
epithelial ovarian cancer or primary peritoneal cancer?
DR LOVE: Can you discuss the data from the GOG-0170D trial evaluating
the use of bevacizumab for patients with persistent or recurrent
epithelial ovarian cancer or primary peritoneal cancer?
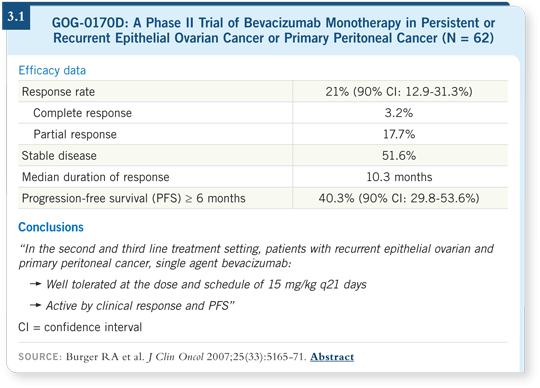
![]() DR BURGER: Among 62 patients who had been previously treated with one or
two cytotoxic regimens and had measurable disease by RECIST, bevacizumab
led to a 21 percent objective response rate and a 40 percent progression-free
survival of at least six months (Burger 2007; [3.1]).
DR BURGER: Among 62 patients who had been previously treated with one or
two cytotoxic regimens and had measurable disease by RECIST, bevacizumab
led to a 21 percent objective response rate and a 40 percent progression-free
survival of at least six months (Burger 2007; [3.1]).
A regression analysis performed at the conclusion of the trial factored in variables associated with disease progression in patients enrolled on Phase II trials of traditional cytotoxic agents: number of prior regimens, time from completion of initial therapy to first recurrence, age and performance status.
None of those factors predicted the time to disease progression in patients treated with bevacizumab (Burger 2007).
![]() DR LOVE: Can you compare GOG-0170D to the other recently reported
Phase II trial of bevacizumab in patients with platinum-resistant ovarian
cancer or peritoneal serous cancer (Cannistra 2007)?
DR LOVE: Can you compare GOG-0170D to the other recently reported
Phase II trial of bevacizumab in patients with platinum-resistant ovarian
cancer or peritoneal serous cancer (Cannistra 2007)?
![]() DR BURGER: In GOG-0170D, the patients had received either one or two
prior regimens. About 40 of the patients had experienced disease progression
within six months of receiving their most recent platinum-containing
regimen, and the remainder had a platinum-free interval greater than or
equal to six months (Burger 2007). The other single-agent bevacizumab trial
required that the patients’ disease be either primarily or secondarily platinum-resistant,
and they could have received two to three prior regimens (Cannistra
2007).
DR BURGER: In GOG-0170D, the patients had received either one or two
prior regimens. About 40 of the patients had experienced disease progression
within six months of receiving their most recent platinum-containing
regimen, and the remainder had a platinum-free interval greater than or
equal to six months (Burger 2007). The other single-agent bevacizumab trial
required that the patients’ disease be either primarily or secondarily platinum-resistant,
and they could have received two to three prior regimens (Cannistra
2007).
So regarding risk of disease progression with any therapy, the patients in the trial reported by Cannistra were at higher risk. In the trial by Cannistra, the objective response rate was 16 percent (Cannistra 2007). In GOG-0170D, the response rate was 21 percent (Burger 2007; [3.1]).
![]() DR LOVE: What was observed in the two studies in terms of side effects and
toxicity?
DR LOVE: What was observed in the two studies in terms of side effects and
toxicity?
![]() DR BURGER: The differences in toxicity were interesting. It’s hard to compare
across trials, but the trial by Cannistra was closed prematurely due to five cases
of gastrointestinal perforation, and GOG-0170D had zero cases of gastrointestinal
perforation.
DR BURGER: The differences in toxicity were interesting. It’s hard to compare
across trials, but the trial by Cannistra was closed prematurely due to five cases
of gastrointestinal perforation, and GOG-0170D had zero cases of gastrointestinal
perforation.
In GOG-0170D, the rate of Grade III/IV proteinuria was minimal. Only one patient developed nephrotic syndrome, and it reversed after the discontinuation of bevacizumab. Approximately 10 percent of the patients had clinically relevant hypertension requiring antihypertensive therapy (Burger 2007). In the trial by Cannistra, no patients developed Grade III/IV proteinuria, but a number of patients with hypertension required therapy. Three patients experienced Grade III/IV arterial thrombotic events (Cannistra 2007).
Track 11
![]() DR LOVE: Can you discuss evolving clinical research on the use of
bevacizumab in combination with IP therapy?
DR LOVE: Can you discuss evolving clinical research on the use of
bevacizumab in combination with IP therapy?
![]() DR BURGER: IP chemotherapy is considered a standard option for patients
with Stage III ovarian cancer, especially those who have undergone optimal
cytoreductive surgery. Three Phase III trials have demonstrated a survival
advantage with IP regimens in combination with IV chemotherapy compared
to standard IV chemotherapy regimens alone (Alberts 1996; Armstrong 2006;
Markman 2001).
DR BURGER: IP chemotherapy is considered a standard option for patients
with Stage III ovarian cancer, especially those who have undergone optimal
cytoreductive surgery. Three Phase III trials have demonstrated a survival
advantage with IP regimens in combination with IV chemotherapy compared
to standard IV chemotherapy regimens alone (Alberts 1996; Armstrong 2006;
Markman 2001).
It’s important to establish the safety and efficacy of these IP cytotoxic regimens in combination with bevacizumab. A Phase III trial is being developed to evaluate modified IP cytotoxic therapy in combination with bevacizumab for patients with Stage III, optimally debulked ovarian cancer.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Tate Thigpen, MD
- Select publications
Ursula A Matulonis, MD
- Select publications
Robert A Burger, MD
- Select publications
Bradley J Monk, MD
- Select publications
Ovarian Cancer Update:
A CME Audio Series and Activity