
 |
|||||

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: What are some of the questions medical oncologists and
gynecologic oncologists ask about the management of newly diagnosed
ovarian cancer?
DR LOVE: What are some of the questions medical oncologists and
gynecologic oncologists ask about the management of newly diagnosed
ovarian cancer?
![]() DR MONK: The standard up-front treatment for epithelial ovarian cancer is
maximal surgical debulking followed by six courses of intravenous platinum- and
taxane-based chemotherapy administered every three weeks. I believe that guidelines have established the platinum drug as carboplatin at an area under
the curve (AUC) between 5.0 and 7.5 mg/mL and the taxane as paclitaxel at a
dose of 175 mg/m2 administered over three hours (NCCN 2008).
DR MONK: The standard up-front treatment for epithelial ovarian cancer is
maximal surgical debulking followed by six courses of intravenous platinum- and
taxane-based chemotherapy administered every three weeks. I believe that guidelines have established the platinum drug as carboplatin at an area under
the curve (AUC) between 5.0 and 7.5 mg/mL and the taxane as paclitaxel at a
dose of 175 mg/m2 administered over three hours (NCCN 2008).
However, questions then emanate and alter that standard. One can outline seven acceptable modifications: (1) using IP chemotherapy, (2) administering more than six cycles of chemotherapy, (3) using weekly chemotherapy, (4) substituting docetaxel as the taxane, (5) adding a targeted agent, specifically bevacizumab, (6) using reassessment surgery when the disease is in remission at the completion of adjuvant chemotherapy to confirm whether the tumor is in remission and (7) continuing chemotherapy during remission as maintenance or consolidation therapy.
Track 4
![]() DR LOVE: What clinical trials are ongoing for patients in the up-front
treatment setting?
DR LOVE: What clinical trials are ongoing for patients in the up-front
treatment setting?
![]() DR MONK: One of the two scientific priorities being evaluated in the up-front
treatment of epithelial ovarian cancer is the incorporation of bevacizumab.
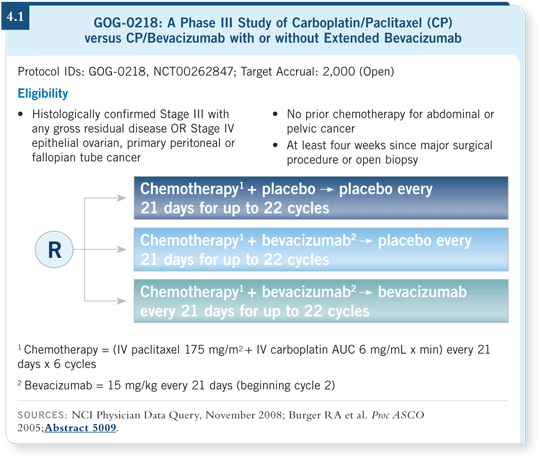
The GOG-0218 trial (4.1) adds bevacizumab to a platinum-and-taxane
backbone. It also addresses maintenance bevacizumab in a third arm, even
when the patient’s disease is in remission.
DR MONK: One of the two scientific priorities being evaluated in the up-front
treatment of epithelial ovarian cancer is the incorporation of bevacizumab.
The GOG-0218 trial (4.1) adds bevacizumab to a platinum-and-taxane
backbone. It also addresses maintenance bevacizumab in a third arm, even
when the patient’s disease is in remission.
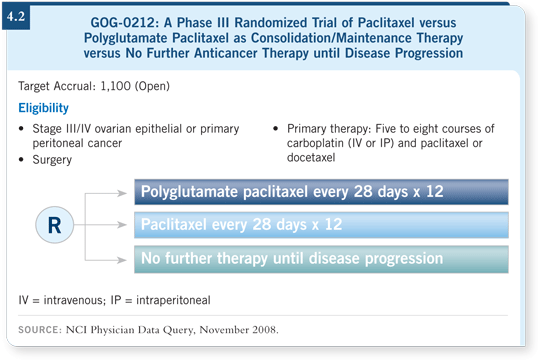
The second scientific priority, also one of my seven controversies, is consolidation therapy. GOG-0212 (4.2) randomly assigns patients who are in remission to no treatment, 12 months of paclitaxel or 12 months of polyglutamate paclitaxel.
Track 8
![]() DR LOVE: Could you discuss your research on trabectedin?
DR LOVE: Could you discuss your research on trabectedin?
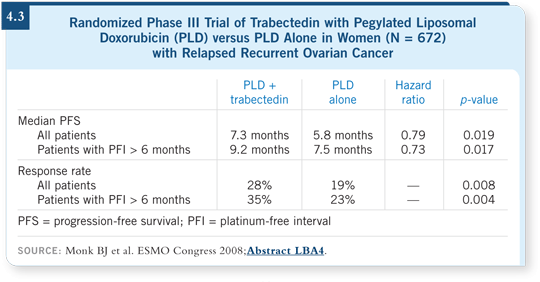
![]() DR MONK: At the 2008 European Society of Medical Oncology meeting,
I presented a trial with trabectedin — a DNA-active drug initially derived
from the Caribbean sea squirt. I evaluated PLD with or without trabectedin
in almost 700 patients. The combination demonstrated an improvement in progression-free survival and response rate. Most of the benefit was observed
in patients with chemotherapy-sensitive disease (Monk 2008; [4.3]).
DR MONK: At the 2008 European Society of Medical Oncology meeting,
I presented a trial with trabectedin — a DNA-active drug initially derived
from the Caribbean sea squirt. I evaluated PLD with or without trabectedin
in almost 700 patients. The combination demonstrated an improvement in progression-free survival and response rate. Most of the benefit was observed
in patients with chemotherapy-sensitive disease (Monk 2008; [4.3]).
Phase II trials of single-agent trabectedin have shown substantial activity in patients with chemotherapy-sensitive disease, with response rates between 30 and 40 percent. In patients with chemotherapy-resistant disease, the response rates were between five and 10 percent (Krasner 2007; Sessa 2005).
Tracks 11-12
![]() DR LOVE: Would you discuss what you know about pertuzumab?
DR LOVE: Would you discuss what you know about pertuzumab?
![]() DR MONK: Pertuzumab is interesting because it is an antibody to HER3,
which seems to be an important biomarker and target in epithelial ovarian
cancer. Pertuzumab activity was demonstrated in the report of a randomized
Phase II study of gemcitabine with or without pertuzumab (Makhija
2007; [2.1, page 9]). The final data from that study reported at ASCO 2008
suggested an association between efficacy and HER3 gene expression levels by
RT-PCR (Amler 2008).
DR MONK: Pertuzumab is interesting because it is an antibody to HER3,
which seems to be an important biomarker and target in epithelial ovarian
cancer. Pertuzumab activity was demonstrated in the report of a randomized
Phase II study of gemcitabine with or without pertuzumab (Makhija
2007; [2.1, page 9]). The final data from that study reported at ASCO 2008
suggested an association between efficacy and HER3 gene expression levels by
RT-PCR (Amler 2008).
That endpoint was exploratory, so we question whether the evidence is sufficient or if we need to validate HER3 as a biomarker in a large prospective study before proceeding with studies of pertuzumab based on that biomarker. We need to study these agents in settings in which they will have the greatest likelihood of being effective, so I believe we should attempt to enrich the patient populations. We should use HER3 as a biomarker now to study pertuzumab. If the results are positive, the study should expand to a broader population.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Tate Thigpen, MD
- Select publications
Ursula A Matulonis, MD
- Select publications
Robert A Burger, MD
- Select publications
Bradley J Monk, MD
- Select publications
Ovarian Cancer Update:
A CME Audio Series and Activity