
 |
|||||

| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: What is your treatment approach for optimally debulked
ovarian cancer?
DR LOVE: What is your treatment approach for optimally debulked
ovarian cancer?
![]() DR MATULONIS: At the Dana-Farber/Harvard Cancer Center, patients with
one centimeter or less of residual tumor after surgery either receive standard
therapy — carboplatin/paclitaxel or IP cisplatin/paclitaxel — or enroll on a
clinical trial.
DR MATULONIS: At the Dana-Farber/Harvard Cancer Center, patients with
one centimeter or less of residual tumor after surgery either receive standard
therapy — carboplatin/paclitaxel or IP cisplatin/paclitaxel — or enroll on a
clinical trial.
We are conducting a pilot study evaluating IP carboplatin/paclitaxel with IV bevacizumab in women with newly diagnosed, optimally cytoreduced carcinoma of Müllerian origin. We also have a Phase II trial in which patients receive IV carboplatin/paclitaxel and bevacizumab for six months and then are randomly assigned to receive bevacizumab alone or bevacizumab in combination with erlotinib.
We have data with this combination in other tumors, and we know single-agent erlotinib has minor activity in recurrent ovarian cancer (Gordon 2005). Synergy may exist between these two biologic agents. In addition, high levels of EGFR expression occur in ovarian cancer cells.
We wouldn’t expect erlotinib to do much for a patient with a large-volume recurrence. However, in the maintenance setting for a patient with small-volume disease, some of the anti-angiogenic effects of erlotinib might combine or perhaps synergize with bevacizumab to be beneficial.
Track 6
![]() DR LOVE: How are you using bevacizumab in your practice?
DR LOVE: How are you using bevacizumab in your practice?
![]() DR MATULONIS: I don’t use bevacizumab for patients with newly diagnosed
ovarian cancer, but I do use it in recurrent disease. I select patients carefully
and counsel them about the risks. I choose patients with a low risk of gastrointestinal
perforation who are not hypertensive. I check their blood pressure and
make sure they don’t have any cardiovascular risk factors.
DR MATULONIS: I don’t use bevacizumab for patients with newly diagnosed
ovarian cancer, but I do use it in recurrent disease. I select patients carefully
and counsel them about the risks. I choose patients with a low risk of gastrointestinal
perforation who are not hypertensive. I check their blood pressure and
make sure they don’t have any cardiovascular risk factors.
![]() DR LOVE: When the ascites in a patient with refractory, advanced disease
responds to bevacizumab, is that an antitumor effect, a vascular effect or both?
DR LOVE: When the ascites in a patient with refractory, advanced disease
responds to bevacizumab, is that an antitumor effect, a vascular effect or both?
![]() DR MATULONIS: When we see a reduction in ascites, we usually see concomitant
decreases in peritoneal metastases, lymph node size and the areas of solid
tumor growth. Although we can’t be 100 percent certain, I believe it’s an
anticancer effect.
DR MATULONIS: When we see a reduction in ascites, we usually see concomitant
decreases in peritoneal metastases, lymph node size and the areas of solid
tumor growth. Although we can’t be 100 percent certain, I believe it’s an
anticancer effect.
Track 9
![]() DR LOVE: What are your thoughts about anti-HER2 therapy in ovarian
cancer, including the potential role of pertuzumab?
DR LOVE: What are your thoughts about anti-HER2 therapy in ovarian
cancer, including the potential role of pertuzumab?
![]() DR MATULONIS: HER2 is not generally overexpressed in ovarian cancer, so we
have to screen many cases to find the few that do overexpress HER2. Even in
those patients with HER2 overexpression, the responses to trastuzumab are not
particularly robust. Pertuzumab works differently than trastuzumab. Trastuzumab
prevents HER2 signaling, while pertuzumab blocks HER2 receptor
dimerization with HER3.
DR MATULONIS: HER2 is not generally overexpressed in ovarian cancer, so we
have to screen many cases to find the few that do overexpress HER2. Even in
those patients with HER2 overexpression, the responses to trastuzumab are not
particularly robust. Pertuzumab works differently than trastuzumab. Trastuzumab
prevents HER2 signaling, while pertuzumab blocks HER2 receptor
dimerization with HER3.
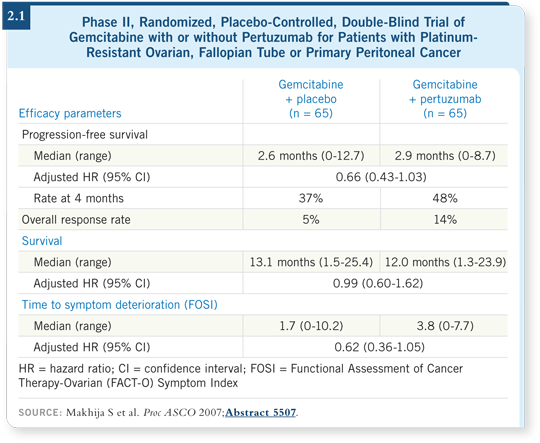
Three trials have evaluated pertuzumab in ovarian cancer. A single-agent study was published in the Journal of Clinical Oncology a number of years ago (Gordon 2006). A European Phase II trial evaluated a platinum-based chemotherapy regimen with or without pertuzumab in patients with platinum-sensitive disease (Kaye 2008). Another Phase II trial compared gemcitabine with or without pertuzumab in platinum-resistant ovarian cancer (Makhija 2007; [2.1]).
Track 10
![]() DR LOVE: Can you summarize the efficacy and toxicity associated with
IP therapy?
DR LOVE: Can you summarize the efficacy and toxicity associated with
IP therapy?
![]() DR MATULONIS: In the GOG-172 trial, comparing IV paclitaxel/cisplatin
to IV paclitaxel with IP cisplatin/paclitaxel in patients with Stage III disease,
the improvement in overall survival of approximately 16 months in the IP
arm was the best we have seen so far (Armstrong 2006; [2.2]). The GOG-111
study, which added IV paclitaxel to IV cisplatin and compared it to IV cisplatin/ cyclophosphamide in patients with Stage III/IV ovarian cancer, reported
an approximately one-year improvement in overall survival (McGuire 1996).
DR MATULONIS: In the GOG-172 trial, comparing IV paclitaxel/cisplatin
to IV paclitaxel with IP cisplatin/paclitaxel in patients with Stage III disease,
the improvement in overall survival of approximately 16 months in the IP
arm was the best we have seen so far (Armstrong 2006; [2.2]). The GOG-111
study, which added IV paclitaxel to IV cisplatin and compared it to IV cisplatin/ cyclophosphamide in patients with Stage III/IV ovarian cancer, reported
an approximately one-year improvement in overall survival (McGuire 1996).
The downside of IP therapy is the toxicity, which is greater than that associated with IV regimens, including increased neuropathy, pancytopenia, nausea, vomiting, renal toxicities and electrolyte problems. The side effects are ameliorated somewhat by reducing the cisplatin dose to 75 mg/m2, but that dose hasn’t been tested in a randomized manner, so it can’t be recommended uniformly for use.

Track 14
![]() DR LOVE: What is your clinical approach for patients with platinum-refractory
ovarian cancer?
DR LOVE: What is your clinical approach for patients with platinum-refractory
ovarian cancer?
![]() DR MATULONIS: Outside of a clinical trial, treatment options include using
one of several cytotoxic agents or bevacizumab. I usually choose either
topotecan or pegylated liposomal doxorubicin (PLD). I do not use the FDA-approved
dose of PLD but a lower dose — 40 mg/m2. The lower dose is more
tolerable for patients in terms of the rash. Generally, if the rash becomes a
problem one can reduce the dose or stretch the interval between cycles. If the
rash is Grade II or worse, I generally drop the dose from 40 to 32 mg/m2.
DR MATULONIS: Outside of a clinical trial, treatment options include using
one of several cytotoxic agents or bevacizumab. I usually choose either
topotecan or pegylated liposomal doxorubicin (PLD). I do not use the FDA-approved
dose of PLD but a lower dose — 40 mg/m2. The lower dose is more
tolerable for patients in terms of the rash. Generally, if the rash becomes a
problem one can reduce the dose or stretch the interval between cycles. If the
rash is Grade II or worse, I generally drop the dose from 40 to 32 mg/m2.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Tate Thigpen, MD
- Select publications
Ursula A Matulonis, MD
- Select publications
Robert A Burger, MD
- Select publications
Bradley J Monk, MD
- Select publications
Ovarian Cancer Update:
A CME Audio Series and Activity